Rasmussen Group
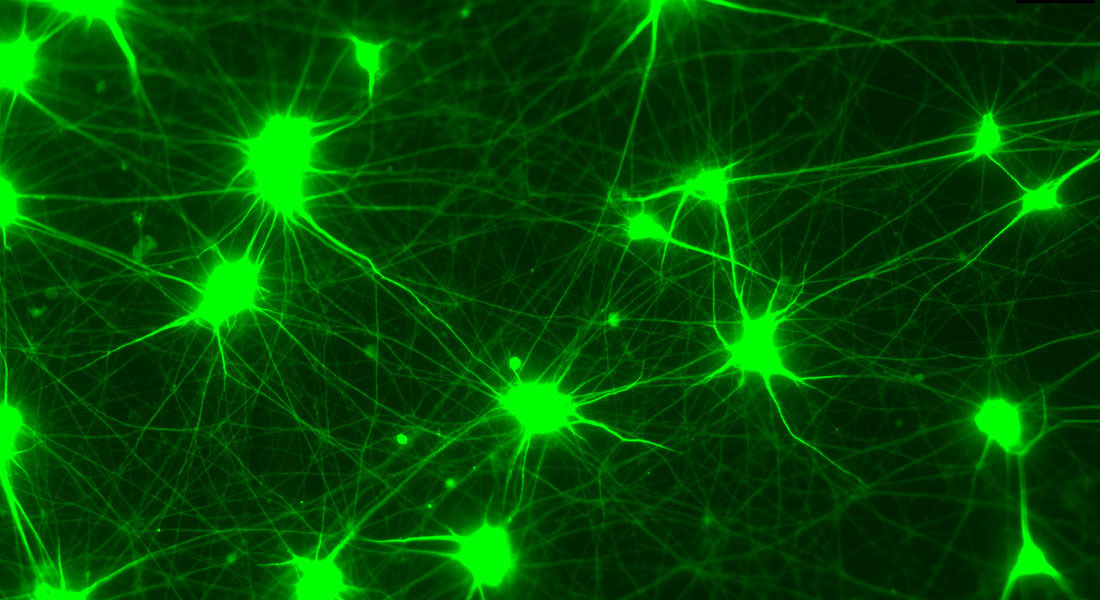
The Rasmussen Group seeks to understand the interconnectedness between the hallmarks of aging and their relative contributions to aging. Our goal is to identify pharmaceutical targets to improve human health during aging.
The correct functioning of these processes is important for avoiding many life-threatening pathologies that can arise because of genetic changes. However, a comprehensive understanding of the scope, dynamics and functions of these complex biological responses is lacking. A particular focus of the group is to elucidate how signaling by unrepaired DNA damage and replication stress promote and regulate mitochondrial function.
“We provide advanced molecular insights into the interplay between cellular pathways that protect against accelerated aging, which is of strong relevance for the design of better treatment strategies for age-related disorders”, says Professor and Group Leader Lene Juel Rasmussen.
The group is excellently positioned to address this challenge due to its expertise in dissection of cellular signaling mechanisms by means of cell biology-, biochemistry- and microscopy-driven approaches. We collaborate with leading experts in human physiology, model organisms and neuroscience, and employ innovative strategies to illuminate the molecular mechanisms promoting healthy aging on both a systems-wide and mechanistic level.
We are researching in the DNA damage response, which is a signaling system that involves a long cascade of cellular processes in response to damage in DNA. DNA damage increases with aging and this may be a result of the gradual attenuation of DNA repair. DNA repair is a maintenance system that surveys the DNA and fixes damage that have accumulated. We discovered that some DNA repair defective diseases have mitochondrial defects.
Key publications for this line of research:
Hassan-Olive et al., 2018, Neurochem. Res.; Fakouri et al., 2017, Nucleic Acids Res.; Martín-
Pardillos et al., 2017, Blood; Thomsen et al., 2017, Neurobiol Aging; Desler et al., 2017, Curr Med Chem; Lauritzen et al., 2015, Am J Physiol Heart Circ Physiol.; Desler et al., 2007, Mutat. Res.; Rasmussen et al., 2003, Nucleic Acids Res.
We have also contributed important research to a basic understanding of the processes that protect and repair the genetic material, and which are central to understanding cancer - how they occur and how to fight them. We identified a novel component of the human mismatch repair (MMR) pathway and our subsequent studies helped elucidate the molecular mechanism of MMR.
We have contributed with pioneering research in the field of molecular biology diagnosis of various types of cancer that are defective in DNA repair. Special mention should be made of the publication in Nature Genetics from 2014, which has contributed with a method for classifying MMR gene variants, which is now being used in clinical settings all over the world.
Key publications for this line of research:
Drost et al., 2018, Genetics in Med.; Liu et al., 2017, Nucleic Acids Res.; Keijzers et al., 2016, Crit Rev Biochem Mol Biol.; Drost et al., 2013, Proc. Natl. Acad. Sci. USA.; Thompson et al., 2014, Nature Genetics; Liberti et al., 2011, DNA Repair; Knudsen et al., 2007, Nucleic Acids Res.; Nielsen et al., 2004, Oncogene; Jäger et al., 2001, Oncogene.
The hallmarks of aging
Nordea-fonden supports a research program on molecular aging that aims to understand the interconnectedness between the hallmarks of aging and their relative contributions to aging, with the final goal of identifying pharmaceutical targets to improve human health during aging.
Role of mitochondrial dysfunction in Alzheimer's Disease: a search for therapeutic strategies
The Olav Thon Foundation has granted money to research on how to explain why defects in DNA repair, and resulting cellular oxidative stress and mitochondrial dysfunction are primary molecular mechanisms in late onset sporadic Alzheimer’s (AD). The study will improve our understanding of the molecular basis of AD, laying the groundwork for effective treatment and/or prevention of AD.
Harnessing the power of Big Data to address the societal challenge of aging
The Novonordisk Foundation has granted money to support a program that (i) delivers novel methods to make optimal use of the available big data across three foci of research, (ii) performs analyses to enhance our ability to explain and predict trajectories of aging and health, and (iii) trains a cadre of physicians, statisticians and life science researchers in the use of big data in biomedicine. To do so, we integrate the systematic exploration of population data with a comprehensive interrogation of biological samples, complemented by experimental validation of data-driven explanatory models.
-
Durhuus JA, Hansson S, Morville T, Kuhlman AB, Dohlmann TL, Larsen S, Helge JW, Angleys M, Muniesa-Vargas A, Bundgaard JR, Hickson ID, Dela F, Desler C, Rasmussen LJ. 2020. Simvastatin improves mitochondrial respiration in peripheral blood cells. Sci Rep. 10(1):17012. doi: 10.1038/s41598-020-73896-2. PMID: 33046789.
-
Navarro JF, Croteau DL, Jurek A, Andrusivova Z, Yang B, Wang Y, Ogedegbe B, Riaz T, Støen M, Desler C, Rasmussen LJ, Tønjum T, Galas MC, Lundeberg J, Bohr VA. 2020. Spatial transcriptomics reveals genes associated with dysregulated mitochondrial functions and stress signaling in Alzheimer's disease. iScience. 23(10):101556. doi: 10.1016/j.isci.2020.101556. eCollection 2020 Oct 23. PMID: 33083725.
-
du Mee DJM, Bak M, Østergaard E, Rasmussen LJ. 2020. Mitochondrial dysfunction induced by variation in the non-coding genome - A proposed workflow to improve diagnostics. Mitochondrion. 53:255-259.
-
Aman Y, Frank J, Lautrup SH, Matysek A, Niu Z, Yang G, Shi L, Bergersen LH, Storm-Mathisen J, Rasmussen LJ, Bohr VA, Nilsen H, Fang EF. 2020. The NAD+-mitophagy axis in healthy longevity and in artificial intelligence-based clinical applications. Mech Aging Dev. Dec 5:111194.
-
Drost M, Tiersma Y, Thompson BA, Spurdle AB, Frederiksen JH, Keijzers G, Pappas L, Boucher KM, Molenkamp S, Zonneveld JBM, van Asperen CJ, Sijmons RH, Goldgar DE, Rasmussen LJ†, Greenblatt MS†, de Wind N†, Tavtigian SV†. 2019. An Integrated, Functional Assay-Based Procedure to Classify Mismatch Repair Gene Variants in Lynch Syndrome. Genet Med.
†Co-corresponding author -
Fakouri NB, Durhuus JA, Regnell CE, Angleys M, Desler C, Hasan- Olive MDM, Martín-Pardillos A, Tsaalbi-Shtylik A, Thomsen K, Lauritzen M, Bohr VA, de Wind N, Bergersen LH, Rasmussen LJ. 2017. Rev1 contributes to proper mitochondrial function via the PARP-NAD+-SIRT1-PGC1α axis. Sci Rep. Oct 2;7(1):12480.
- Martín-Pardillos A, Tsaalbi-Shtylik A, Chen S, Lazare S, van Os RP, Dethmers-Ausema A, Fakouri NB, Bosshard M, Aprigliano R, van Loon B, Salvatori DCF, Hashimoto K, Dingemanse-van der Spek C, Moriya M, Rasmussen LJ, de Haan G, Raaijmakers MHG, de Wind N. 2017. Genomic and functional integrity of the hematopoietic system requires tolerance of oxidative DNA lesions. Blood. Sep 28;130(13):1523-1534.
- Thomsen K, Sherazi N, Yokota T, Hasan-Olive M, Fakouri NB, Desler C, Regnell C, Rasmussen LJ, Dela F, Bergersen LH, Lauritzen M. Initial brain aging: heterogeneity of mitochondrial size is associated with respiratory dysfunction in cortex and hippocampus. 2017. Neurobiol Aging. Aug 12. pii: S0197-4580(17)30259-2.
- Strickertsson J, Desler C, Rasmussen LJ. 2017. Bacterial infection increases risk of carcinogenesis by targeting mitochondria. Semin Cancer Biol. Jul 25. pii: S1044-579X(17)30192-X.
- Liu D, Keijzers G, Rasmussen LJ. 2017. DNA mismatch repair and its many roles in eukaryotic cells. Mutat Res Rev. 773:174-187.
-
Liu D, Frederiksen JH, Liberti SE, Lützen A, Keijzers G, Pena-Diaz J, Rasmussen LJ. 2017. Human DNA polymerase delta double-mutant D316A E318A interferes with DNA mismatch repair in vitro. Nucleic Acids Res. Sep 19;45(16):9427-9440.
-
Lopez-Contreras AJ, Specks J, Barlow JH, Ambrogio C, Desler C, Vikingsson S, Rodrigo-Perez S, Green H, Rasmussen LJ, Murga M, Nussenzweig A, Fernandez-Capetillo O. Increased Rrm2 gene dosage reduces fragile site breakage and prolongs survival of ATR mutant mice. Genes & Dev. 29: 690-695. 2015.
- Thompson BA, Spurdle AB, Plazzer J-P, et al. Application of a five-tiered scheme for standardized classification of 2,392 unique mismatch repair gene variants lodged on the InSiGHT locus-specific database. Nature Genetics. 46: 107-115. 2014.
- Maynard S, Keijzers G, Gram M, Desler C, Bendix L, Budtz-Jørgensen E, Molbo D, Croteau DL, Osler M, Stevsner T, Rasmussen LJ, Dela F, Avlund K, Bohr VA. Relationships between human vitality and mitochondrial respiratory parameters, reactive oxygen species and dNTP levels in peripheral blood mononuclear cells. Aging. 5: 850-864. 2014.
- Strickertsson JAB, Desler C, Rasmussen LJ. Impact of bacterial infections on aging and cancer: Impairment of DNA repair and mitochondrial function of host cells. Exp Gerontology. 56: 164-174. 2014.
- Machado AM, Desler C, Bøggild S, Strickertsson JA, Hansen LF, Figueiredo C, Seruca R, Rasmussen LJ. Helicobacter pylori infection affects mitochondrial function and DNA repair, thus, mediating genetic instability in gastric cells. Mech. Aging Dev. 134: 460-466. 2013.
- Drost M, Lützen A, van Hees S, Ferreira D, Calléja F, Zonneveld JBM, Nielsen FC, Rasmussen LJ, de Wind N. Genetic screens for the identification of pathogenic gene variants in the cancer predisposition Lynch syndrome. Proc. Natl. Acad. Sci. USA. 110: 9403-9408. 2013.
- Andersen SD, Liberti SE, Lützen A, Drost M, Bernstein I, Nilbert M, Dominguez M, Nyström M, Hansen TVO, Christoffersen JW, Jäger AC, de Wind N, Nielsen FC, Tørring PM, Rasmussen LJ. Functional characterization of MLH1 missense variants identified in Lynch Syndrome patients. Human Mutat. 33: 1647-1655. 2012.
- Rasmussen LJ, Heinen CD, Royer-Pokora B, Drost M, Tavtigian S, Hofstra RMW, de Wind N. Pathological assessment of mismatch repair gene variants in Lynch syndrome: past, present and future. Human Mutat. 33: 1617-1625. 2012.
- Machado AMD, Figueiredo C, Touati E, Máximo V, Sousa S, Michel V, Carneiro F, Nielsen FC, Seruca R, Rasmussen LJ. Helicobacter pylori infection induces genetic instability of nuclear and mitochondrial DNA in gastric cells. Clin. Cancer Res. 15: 2995-3002. 2009.
- Desler C, Petersen BM, Stevnsner T, Matsui S-I, Kulawiec M, Singh KK, Rasmussen LJ. Mitochondria as determinant of nucleotide metabolism and chromosomal stability. Mutat. Res. 625:112-124. 2007.
Group leader
Lene Juel Rasmussen
Executive Director, professor
lenera@sund.ku.dk
Phone: +45 35 32 67 17
Mobile: +45 29 17 65 32
Group members
| Name | Title | Phone | |
|---|---|---|---|
| Linda Boje Dalsgaard | Laboratory Technician | +4535327813 | |
| Yuan Li | Assistant Professor | +4535328139 | |
| Zhiquan Li | Postdoc |



